LANDMARK TRIALS IN
Renal Artery Stenosis
First Posted on RFN: April 17, 2019
Reviewed: November 2020
Renal artery stenosis (RAS) can lead to a reduction in kidney blood flow with resultant ischemia of the affected kidney. The consequences of this reduction are a decline in estimated glomerular filtration rate (eGFR) and activation of the renin-angiotensin-aldosterone system (RAAS) with the development of renovascular hypertension. Historically, stenting of RAS was believed to reverse the decreased blood flow and lessen the activation of the RAAS. The most common cause of RAS is atherosclerotic renal artery stenosis (ARAS), which is responsible for up to 90% of the cases of renovascular disease. The majority of the remaining cases were due to fibromuscular dysplasia. Below we will describe the landmark trials that provide the evidence for optimal treatment for our patients with RAS and are focused on two outcomes; (1) stabilization or improvement in GFR (renal preservation) and (2) blood control.
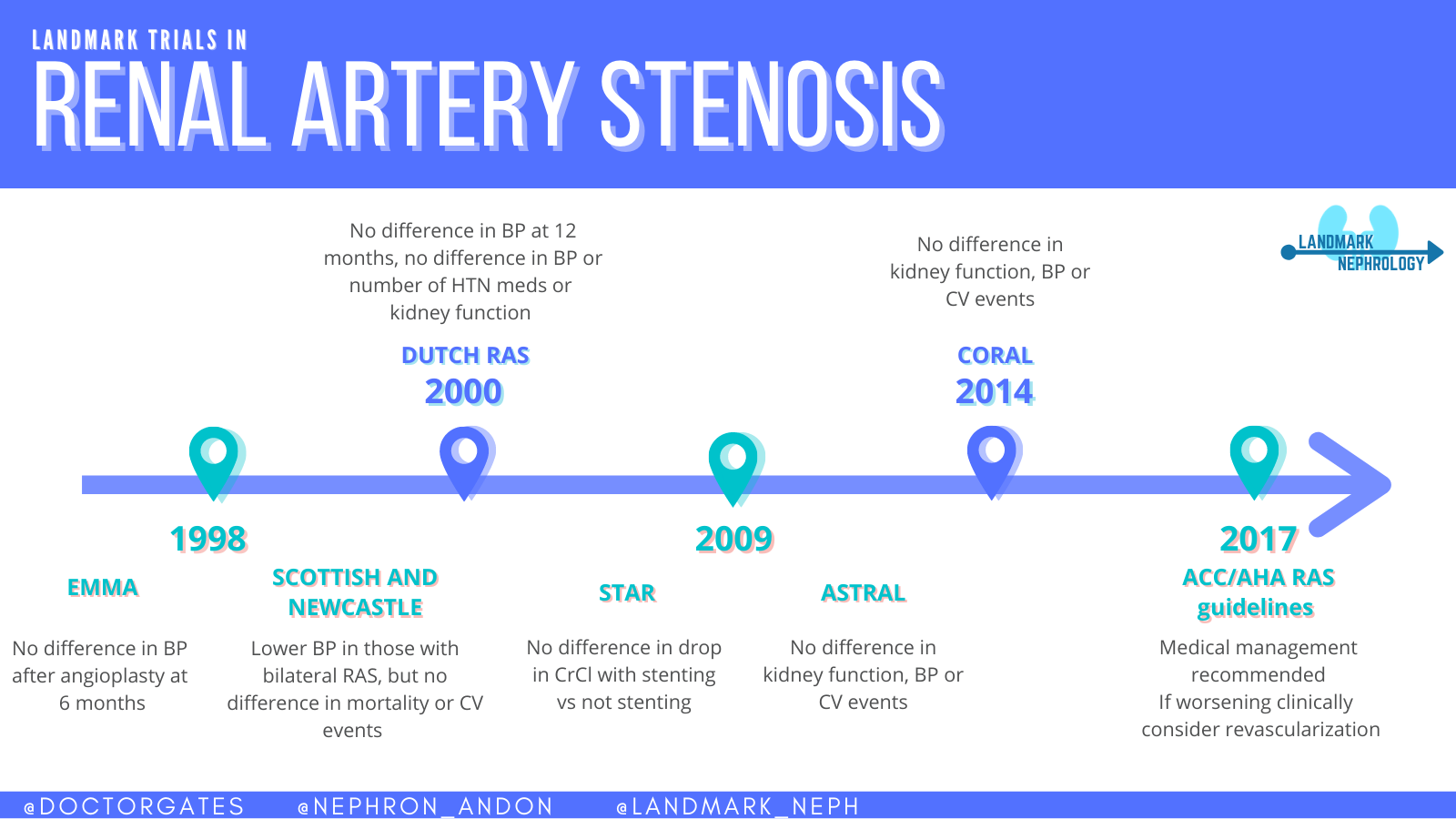
A few small randomized trials began in the late 1990’s to determine a whether angioplasty was superior to medical therapy for blood pressure control due to RAS. The EMMA study group looked at 49 total patients, but was not able to find any improvements in blood pressure with angioplasty at six months. Similarly the Scottish and Newcastle collaborative group in the same year did not find any difference with angioplasty in a total cohort of 135 patients with the longest follow up of 54 months. A Dutch group also looked at 106 patients randomized to medical therapy versus angioplasty. At 12 months there no differences in systolic blood pressure, numbers of medication, or changes in kidney function. All of these trials had limitations of short time frames, high (29% average) crossover rate, and small cohorts that may have lacked power to adequately determine differentiation.
Let’s review three important randomized trials below.
1. STAR 2009
The beginning of quality randomized clinical trials looking at RAS began in 2009. The STAR trial was focused on preservation of renal function and was published in the Annals of Internal Medicine. This was a randomized trial based in the Netherlands and France examining 140 patients with eGFR <80 mL/min/1.73 m2, stable blood pressure <140/90 mmHg, and ostial RAS of at least 50% stenosis. These patients were randomized to medical therapy including anti-hypertensive agents, atorvastatin, and aspirin, or to the same medical therapy along with stenting. After 24 months, the authors did not find a significant difference between the groups (primary outcome: a 20% or greater decrease in creatinine clearance). Ten of the 64 patients (16%) in the stent placement group and 16 patients (22%) in the medical treatment only group reached the primary end point (hazard ratio, 0.73 [95% CI, 0.33 to 1.61]. The trial had limitations with 25% of the stenting arm that never actually received a stent. The STAR trial was criticized for its small cohort and exclusion of high risk RAS patient as it is recognized that decreased kidney blood flow and clinical consequences of RAS do not occur until there is greater than 70-80% lumen narrowing.
2. ASTRAL
Also published in 2009, this trial was larger in size than STAR with enrollment including 806 patients. After a mean follow up of 34 months, there was no significant difference in kidney outcomes, blood pressure control, or cardiovascular events (p=0.06). This study had several limitations as well. Not only did 40% of enrollees have < 70% stenosis, but 25% had normal eGFR who would be considered very low risk and not likely to benefit from stenting in the first place. The major criticism of ASTRAL was allowing physicians to only enroll patients if they felt that revascularization would be beneficial. Because of a lack of equipoise there was a clear recruitment bias as evidenced by the low rate of randomization of otherwise eligible patients. As with the STAR trial, ASTRAL was criticized for not including enough high-risk patients who would benefit from revascularization.
3. CORAL
The largest RAS trial to date with comparison of cardiovascular and kidney events in patients with ARAS was the Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial published in NEJM 2014. This trial was designed to address some of the limitations and criticisms of the previous studies. A large cohort of 947 patients was included and all had a systolic blood pressure > 155 mmHg, were on more than two anti-hypertensive medications, and had an eGFR less than 60 mL/min/1.72 m2. The patients were randomized to medical therapy, or stenting with medical therapy, for a median of 43 months. The primary endpoint was a composite of death from cardiovascular or renal causes, stroke, myocardial infarction, hospitalization for congestive heart failure, progressive loss of eGFR, or need for permanent dialysis. The trial was longer than both STAR and ASTRAL and contained a higher risk population, addressing the criticisms seen of previous studies. Despite the large size and enrollment of high-risk patients, there was no difference in occurrence of the primary composite endpoint or any of the individual components. The only significant finding was lower systolic blood pressure in the stenting arm at the end of the trial (difference of -2.3mmHg, 95% CI; p=0.03). A correspondence in NEJM was published four months later that looked at patients with uncontrolled hypertension.
When examining the three randomized trials of STAR, ASTRAL and CORAL, there was no evidence of clinical benefit by revascularization with stenting/angioplasty above medical therapy in those with ARAS. All three trials have been criticized for not including enough high-risk patients.
A meta-analysis on the subject was also completed. It combined data from these 3 major articles in addition to smaller trials. In total, it combined data from 2222 patients. In the analysis of positive trials for changes in diastolic BP it demonstrated that this benefit is quite small (2.0 mmHg ‐2.00 mmHg; 95% CI ‐3.72 to ‐0.27) and there was no benefit on decreasing systolic BP. There was also no difference in kidney function measure by Cr. The decrease in antihypertensive medications in the intervention groups was overall also quite small (MD ‐0.18; 95% CI ‐0.34 to ‐0.03). There were no increase in adverse events in the intervention groups when compared to the medical management group.
Our medical societies have also come to some recommendations based on these major trials. The 2017 ACC/AHA Hypertension Guidelines have addressed ARAS and treatment guidance. The writers recommend at minimum all patients with ARAS should have medical therapy citing Level 1A evidence. If a patient has failed medical management and continues to have clinical evidence of refractory hypertension, worsening renal function or intractable heart failure, revascularization should be considered. ACC/AHA do not fully endorse any specific procedure or evidence of outcome benefit.
There are currently several clinical trials looking at devices and other medical treatments to help determine who should benefit from intervention.
Author: Gates Colbert, MD, FASN
Edited by: Bradley Denker, MD.
ARTICLES
- Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group
- Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group
- The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group
- Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial.
- Revascularization versus Medical Therapy for Renal-Artery Stenosis.
- Stenting and Medical Therapy for Atherosclerotic Renal-Artery Stenosis.
- ACC/AHA RAS guidelines
