LANDMARK TRIALS IN
RRT
Landmark Nephrology: Renal Replacement Therapy (RRT) Timing in Critically Ill Patients
First Posted on RFN: August 23, 2019
Updated on: December 2020
Currently, the timing of initiation of renal replacement therapy (RRT) in critically ill patients with acute kidney injury (AKI) remains controversial. Despite several randomized controlled trials (RCTs) comparing early versus late initiation strategies of RRT, the results are ambiguous (and the definitions of “early” and “late” are often different from trial to trial). Let’s review some of the landmark studies in this area.
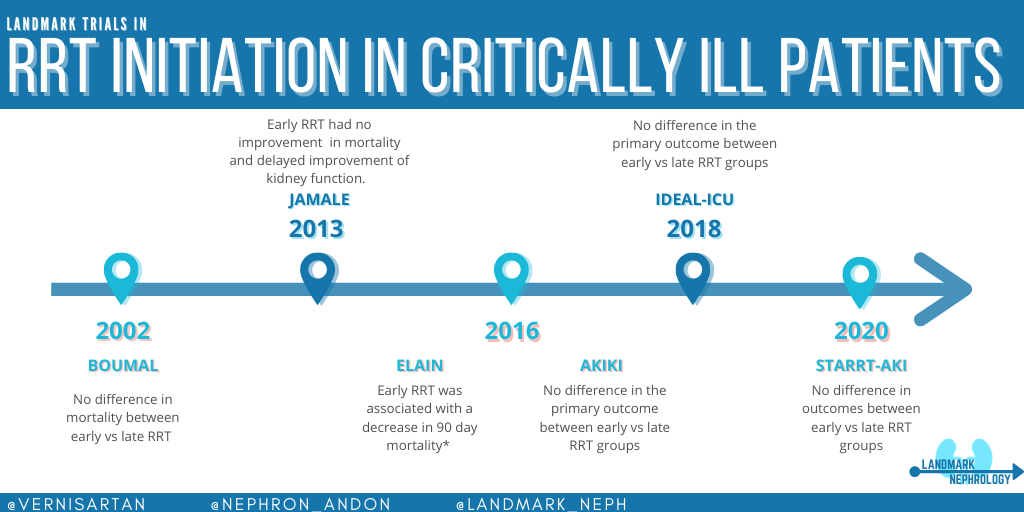
First, let’s examine the data that have led up to the modern-day trials of ELAIN, AKIKI, and IDEAL-ICU. In the table below, we summarize 3 important RCTs from 2002 – 2013.
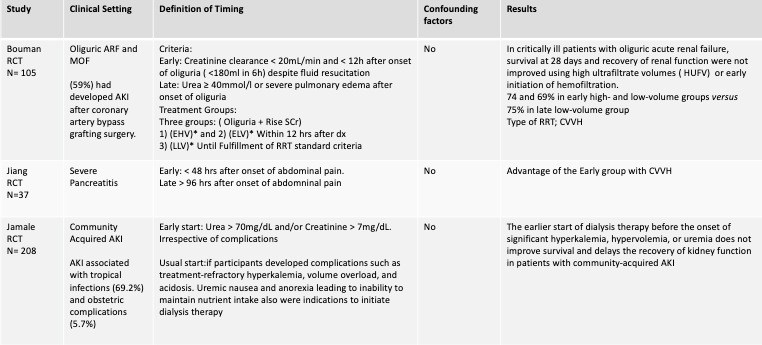
Table 1. Summary of 3 randomized control trials comparing “early” vs “late” initiation of renal replacement therapy (Bouman 2002 , Jiang 2005, Jamale 2013; EHV: Early high-volume hemofiltration; ELV: Early low-volume hemofiltration; LLV: Late low-volume hemofiltration)
Though some retrospective studies favored “early” start of RRT, the RCTs above did not show any clear benefit. Importantly, the definition of “early” vs “late” was heterogeneous among these small studies. We’ll now review the more recent RCTs: ELAIN, AKIKI, IDEAL-ICU, and STARRT-AKI.
1. ELAIN (JAMA)
In 2016, Zarbock et al in Germany evaluated the effect of Early vs. Delayed initiation of RRT on mortality in critically ill patients with AKI. 93.5% of patients had AKI after surgery, of which 46.75% were cardiac surgery patients. This study demonstrated a significant reduction in 90-day mortality with the EARLY strategy (AKI KDIGO 2), in a group of almost entirely surgical patients.
Patients were divided into two groups for the initiation of RRT:
-
EARLY: Within 8 hours of satisfying the criteria for AKI KDIGO stage 2 and a Neutrophil Gelatinase-Associated Lipocalin (NGAL) > 150 ng/ml.
-
DELAYED: Within 12 hours of either satisfying the criteria for AKI KDIGO stage 3 or an absolute indication for RRT.
Nearly 75% of patients (in both groups) developed fluid overload, 88% of needed catecholamines >0.1 µg/kg/min as a vasopressor prior to initiation of RRT, and the first-line RRT modality was continuous venovenous hemodiafiltration (CVVHDF).
There were significant weaknesses in this study:
(1) Fragility Index of 3 – this means that a shift of only 3 patients from the event to non-event group would render the findings statistically insignificant.
(2) ELAIN was a single-center study in Europe and not all the treatments were standardized between groups. Treatments were unblinded by the nature of starting the treatment, possibly introducing bias and challenging its internal validity.
2. AKIKI (NEJM)
Also in 2016, Gaudry et al published the multi-centered AKIKI trial. They defined early vs. delayed RRT as:
-
EARLY: within 6 hours from randomization after satisfying criteria for AKI KDIGO stage 3.
-
DELAYED: RRT was initiated if the patient developed AKI KDIGO 3 plus urgent/emergent dialysis indication
The choice of modality, duration, and interval between sessions of RRT was left to the discretion of each study site and was prescribed and monitored according to national guidelines. As a result, over 50% received intermittent RRT.
AKIKI had more critically ill patients with severe AKI than the ELAIN trial. Unlike the ELAIN study, the main reason for ICU admission was a medical rather than a surgical indication (~66% septic shock in both groups, 20% surgical cases). The primary outcome showed no significant difference in mortality between early strategy and delayed strategy of RRT. Over 50% of patients received the initial modality of intermittent hemodialysis (HD), despite the fact that 85% of patients required vasopressor support upon initiation of RRT.
A major weakness of this study was that it only included patients with advanced kidney injury, and therefore the results may not be generalizable to patients with less severe AKI. Also, like ELAIN, this trial was not blinded by the nature of the treatment.
3.IDEAL-ICU (NEJM)
The results between ELAIN and AKIKI were divergent with key differences, and they did not fully answer the question of the ideal timing of RRT initiation. Two years later in 2018, Barbar and colleagues published the IDEAL-ICU trial. This RCT importantly included more patients with sepsis than ELAIN and AKIKI which is clinically relevant as it is the number one cause of AKI in hospitalized patients.
The authors defined early versus delayed initiation of RRT as:
-
EARLY: RRT was started within 12 hours after the onset of AKI RIFLE Stage F.
-
DELAYED: RRT was initiated after a delay of 48 hours of AKI RIFLE Stage F
The choice of RRT technique (intermittent or continuous) was at the discretion of clinical experts at each site.
Continuous slow therapy was used as initial modality in 57% of the patients assigned to the early strategy group, and in 55% of those in the delayed group (p = 0.62).
In the delayed strategy group, 29% of the patients did not require RRT because they had spontaneous recovery of renal function. This lead to a difference in actual receipt of RRT with 97% in early group vs. 62% in the delayed group (p=0.001). Nonetheless, 17% (41 patients) of the late strategy group developed an absolute criterion for the initiation of RRT before reaching 48-hours after the diagnosis of AKI RIFLE stage F. The mortality of these patients was 68%, higher than the general mortality (55%).
This trial showed no statistically significant difference in the primary outcome (mortality) between early versus late initiation of RRT patients with septic shock and severe AKI.
4. STARRT-AKI (NEJM)
This much-anticipated trial was published in July 2020 boasting the largest participant total yet (n=2927) in 15 countries across 4 continents. Patients were determined to be “provisionally eligible” if they were admitted to the ICU with AKI (KDIGO Stage 2 or 3) or developed severe AKI (defined as a doubling of serum creatinine from baseline, Cr ≥4 mg/dL, or urine output was less than 6 cc/kg in the previous 12 hours), and did NOT have baseline GFR<20 ml/min, emergent dialysis indications, or certain etiologies of AKI. Randomization occurred once the responsible physician made the determination that immediate initiation of RRT was not required and therefore was “fully eligible” for inclusion in the study.
The authors defined early versus delayed initiation of RRT as:
-
EARLY / ”ACCELERATED”: RRT started within 12 hours, once determined to be “fully eligible” (median time = 6.1 hours)
-
DELAYED / ”STANDARD”: RRT not started until one of the following criteria were met – K+ ≥6 mEq/L, pH ≤ 7.2, HCO3– ≤ 12 mEq/L, PaO2/FiO2 ≤ 200 + Volume overload, persistent AKI for 72 hours after randomization, once determined to be “fully eligible” (median time = 31.1 hours)
The choice of RRT technique (intermittent or continuous) was once again at the discretion of clinical experts at each site (CRRT ~70%, IHD ~25%, SLED/PIRRT ~5%)
The primary outcome of death was virtually identical in both groups (43.9% in accelerated group vs. 43.7% in standard group), despite patients in the standard treatment group having higher SOFA scores, lower hemoglobin, and a more concerning electrolyte panel. Additionally, no significant differences were found in the composite outcomes, which included death at the 28-day mark, length of hospitalization, RRT or ventilator-free days, discharge from the ICU or hospital, and quality of life.
None of the pre-specified subgroup analyses of ICU admission type (surgical vs. medical), geographic region, estimated baseline GFR (cut off of 45), and the presence or absence of sepsis, showed any significant differences between the accelerated vs. standard cohort.
For an excellent summary of the recent landmark trials that have tried to settle the question of whether early dialysis for critically ill patients with AKI improves outcomes, check out this comparison table from NephJC:
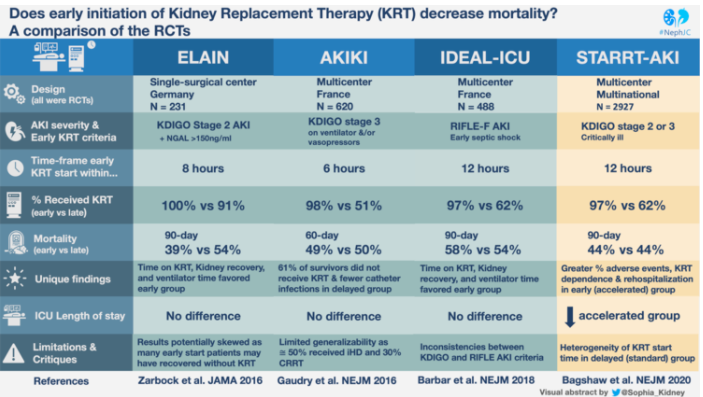
According to clinicaltrials.gov, the AKIKI 2 trial was concluded in March 2020. The investigators of the original AKIKI trial will compare the prior “delayed strategy” (now be considered “standard”) with a strategy that delays RRT for a longer period in the absence of a life-threatening complication. This new delayed strategy will be very similar to that in the STARRT-AKI trial.
Conclusions:
Most would agree that the modern RCTs that have sought to settle the question of whether early RRT improves outcomes in critically ill patients with AKI have shown increasingly convincing evidence that earlier is NOT better. Comparing each RCT is challenging, due to different causes of acute kidney injury in their populations as well as definition of AKI and “early” versus “late” initiation of RRT. As more RCTs converged on the similar conclusions, perhaps we will be back where we started – initiate RRT when you feel the patient requires it (but no sooner).
Post by:
Cristhian Muñoz Menjivar
A.Verner Venegas Vera
Hugo Cesar Campos Gil
Gates B Colbert
Revised and updated by: Jeffrey William