LANDMARK TRIALS IN
Induction Immunosuppression in Kidney Transplantation
Atgam in Induction
OKT3 in Induction
Prospective cohort with OKT3 vs no OKT3
RCT - Atgam vs. OKT3
RCT - ATG vs. no induction
RCT - ATG vs. Atgam in induction
RCT ATG pre-reperfusion vs. 6h post-reperfusion
Almetuzumab induction and prednisone-free maintenance
RCT - ATG vs. Basiliximab in induction
ATG dose optimization in high risk KT
RCT - ATG vs. Daclizumab
Cochrane Review of IL2RA in induction
RCT - Almetuzumab induction vs. rabbit ATG or basiliximab
Almetuzumab vs. rATG followed by rapid steroid taper
rATG vs. IL2RA vs. no induction in HIV+
Cochrane Review of polyclonal and monoclonal antibodies for induction therapy
Legend:
![]()
![]() RCT with >= 2000 patients
RCT with >= 2000 patients
![]() RCT with 1000-1999 patients
RCT with 1000-1999 patients
![]() RCT with 500-999 patients
RCT with 500-999 patients
![]() RCT with < 499 patients
RCT with < 499 patients
![]() Pathophysiology
Pathophysiology
![]() Disease Outcomes and Trajectory
Disease Outcomes and Trajectory
![]() Biomarkers
Biomarkers
[insert logo] Retrospective Cohort
[insert logo] Prospective Cohort
Date: 1967
Abbreviation: Atgam [Prospective Logo]
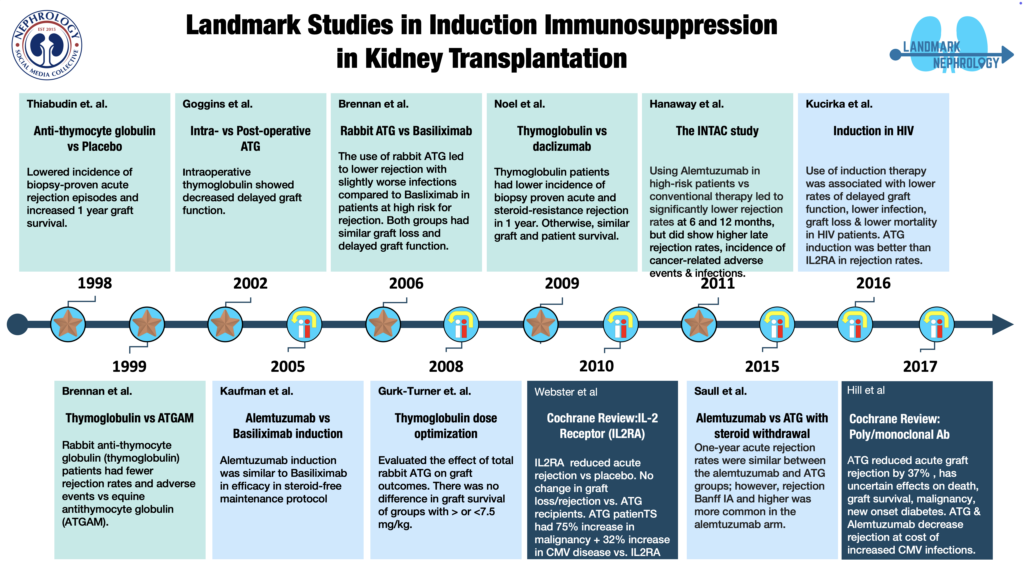
Horse anti human-lymphoid globulin was used for kidney transplantation in humans for the first time in 11 patients (8 who had new kidney transplantation and 3 who had failing grafts) who received equine antilymphocyte globulin in combination with azathioprine and prednisone. The need for therapy with the standard immunosuppressive drugs could be reduced safely in those receiving horse ALG and 2 of the 3 patients with failing graft had stabilization of renal function.
Date: 1985
Abbreviation: OKT3 [Prospective Logo]
Prophylactic treatment of allograft recipients with a monoclonal anti T3+ cell antibody*
OKT3 was used prophylactically in 30 patients along with azathioprine and corticosteroids for 30 days after transplantation. There was excellent graft survival of 90% at 1 year post-transplantation.
*historical reference with no link available
Date: 1989
Abbreviation: Prophylactic OKT3 [Prospective Logo]
Prophylactic use of OKT3 in immunologic high-risk cadaver renal transplant recipients.
Prospective study where prophylactic OKT3 was administered to 27 immunologic high-risk cadaver renal transplant recipients (multiple transplant and/or panel reactive antibody greater than 50%) as part of a sequential immunosuppressive protocol consisting of OKT3, prednisone, azathioprine, and cyclosporine. Patient survival was 100% with a mean follow-up period of 13 months. Graft survival at 1 year was 70%. Prophylactic use of OKT3 in this patient population resulted in superior graft survival when compared with a similar group of patients not receiving OKT3.
Date:1992
Abbreviation: Atgam vs OKT3
Antilymphocyte globulin versus OKT3 induction therapy in cadaveric kidney transplantation: a prospective randomized study 
Single center RCT which randomized 140 patients undergoing deceased donor kidney transplantation to equine antilymphocyte globulin or OKT3. The primary outcome was acute rejection. Both groups received low-dose cyclosporine and steroids. The incidence of acute rejection during the first 3 months after transplantation was similar in the two groups (15% in ALG vs 19% in OKT3, p=0.6). The 3-year actuarial graft survival was comparable in the two arms.
Date: 1998
Abbreviation: Thymoglobulin
Single center randomized controlled trial from France. It included 89 sensitized patients (panel reactive antibodies >5%), undergoing live or deceased donor transplantation randomized to either receive low dose antithymocyte globulin [ATG- 1.25 mg/kg/day for 10 days] or no induction before transplantation. Maintenance agents used were cyclosporine, azathioprine, and steroids. Primary efficacy outcome was the occurrence of acute rejection. ATG induction lowered the incidence of biopsy-proven acute rejection episodes from 64 to 38% (p=0.02). It also increased the 1 year graft survival from 76 to 89% and was associated with a higher 1 year inulin clearance (37+/-15 vs 49+/-18 ml/min). ATG associated side effects included leucopenia and thrombocytopenia.
Date: 1999
Abbreviation: Thymoglobulin vs. Atgam
Single center randomized controlled trial in which 72 patients undergoing live or deceased donor kidney transplantation were randomized to receive rabbit antithymocyte globulin (thymoglobulin 1.5 mg/kg/d x 7d) or equine antithymocyte globulin (Atgam 15 mg/kg/d x 7d) in a 2:1 ratio. Maintenance agents used were cyclosporine or tacrolimus, azathioprine, and steroids. The primary endpoints were the rates of acute rejection patient survival, and graft survival at 6 and 12 months. The incidence of CMV disease and serious adverse events in each arm were also primary endpoints. At 1 year post-transplantation, 4% of thymoglobulin-treated patients experienced acute rejection compared with 25% of Atgam-treated patients (P=0.014). Patient survival was not different, but the composite endpoint of freedom from death, graft loss, or rejection, the “event-free survival,” was superior to thymoglobulin (94%) compared with Atgam (63%; P=0.0005). Fewer adverse events occurred with thymoglobulin (P=0.013) but no difference in rates of SAE (more leucopenia in thymo vs Atgam). The incidence of cytomegalovirus disease was lesser with thymoglobulin than Atgam at 6 months (10% vs. 33%; P=0.025)
Date: 2002
Abbreviation: ATG Intraop vs. Postop
Single center randomized controlled trial which compared intraoperative (before reperfusion) versus postoperative (6 hours after reperfusion) thymoglobulin in 58 recipients undergoing deceased donor transplantation patients. Baseline immunosuppression consisted of tacrolimus or cyclosporine A, steroids, and mycophenolate mofetil. All patients received 1mg/kg ATG for 3-6 days after the first dose as specified above. The study compared allograft function during the initial postoperative period and then monthly until 6 months. Delayed graft function(DGF) was defined as the need for dialysis in the first week post-transplant. Intraoperative thymoglobulin administration was associated with significantly less DGF and a lower mean serum creatinine on postoperative days 10 and 14 (P <0.05). Posttransplant length of stay was also significantly shorter for the intraoperative thymoglobulin patient group. The acute rejection rate was also lower in the intraoperative treatment group but this did not achieve statistical significance
Date: 2005
Abbreviation: Alemtuzumab
Alemtuzumab induction and prednisone-free maintenance immunotherapy in kidney transplantation: comparison with basiliximab induction-long-term results. [Retrospective logo]
Single-center, nonrandomized, retrospective, sequential study design to evaluate outcomes in alive or decreased kidney transplant recipients given either alemtuzumab (n = 123) or basiliximab (n = 155) induction in combination with a steroid-free maintenance protocol using tacrolimus and mycophenolate mofetil. There was no significant difference in patient and graft survival rates between the two groups. Acute rejection was less common in alemtuzumab arm in less than 3m period but comparable with basiliximab at 1 year.
Date: 2006
Abbreviation: ATG vs. Basiliximab
Rabbit ATG vs Basiliximab in Renal Transplantation ![]()
Multicenter randomized controlled trial in which rabbit ATG versus basiliximab was studied as an induction agent in deceased donor kidney transplantation of 278 patients who were at high risk of acute rejection or delayed graft function. The primary endpoint was a composite of acute rejection, delayed graft function, graft loss, and death. At 12 months, the incidence of the composite endpoint was similar in the two groups (P=0.34). The antithymocyte globulin group had lower incidences of acute rejection with similar incidences of graft loss, delayed graft function, and death. Patients in the ATG arm had a greater incidence of infection but a lower incidence of cytomegalovirus disease
Date: 2008
Abbreviation: ATG Optimization
Thymoglobulin dose optimization for induction therapy in high risk kidney transplant recipients [Retrospective logo]
A retrospective cohort study to evaluate the effect of total dose of rabbit ATG on graft outcomes of 96 adult high immunological risk kidney transplant recipients undergoing live or deceased donor transplantation patients. Patients were maintained on tacrolimus, mycophenolate mofetil, and steroids. Group 1 (n=33) received ≤ 7.5mg/kg rATG and group 2 (n=63) received > 7.5 mg/kg rATG. Graft and patient survival, incidence of acute rejection, and 12-month serum creatinine were examined. There was no significant difference in graft survival, incidence of BPAR at 1 year, and serum creatinine at 1 year between the two groups.
Date: 2009
Abbreviation: ATG vs. Daclizumab
Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. ![]()
Multicentre randomized controlled trial which randomized 227 high immunological risk renal transplant recipients to Daclizumab (5 mg/kg x 5 doses every 15d) or antithymocyte globulin (1.25 mg/kg/d x 8 d). The primary outcome was the proportion of patients with biopsy-proven acute rejection (BPAR) at 1 year. Patients treated with thymoglobulin had a lower incidence of both biopsy-proven acute rejection (15.0% versus 27.2%; P = 0.016) and steroid-resistant rejection (2.7% versus 14.9%; P= 0.002) at one year. One-year graft and patient survival rates were similar between the two groups.
Date: 2010
Abbreviation: IL2RA
Cochrane Database Syst Rev. Interleukin 2 receptor antagonists (IL2RA) for kidney transplant Recipients [Retrospective logo]
Cochrane database review included 71 studies (306 reports, 10,537 participants). As compared to placebo, IL2Ra reduced graft loss, including death with a functioning graft, by 25% at six months & one year. At one year biopsy-proven acute rejection BPAR was reduced by 28% & CMV disease was reduced by 19%. When IL2Ra were compared to ATG, there was no difference in graft loss at any time point, or for acute rejection diagnosed clinically, but there was benefit of ATG therapy over IL2Ra for biopsy-proven acute rejection at one year. There was a 75% increase in malignancy & a 32% increase in CMV disease in ATG compared to IL2RA. ATG patients experienced significantly more fever, cytokine release syndrome, and other adverse reactions to drug administration and more leucopenia. There were no apparent differences between basiliximab and daclizumab.
Date: 2011
Abbreviation: INTAC
Alemtuzumab induction in renal transplantation ![]()
Multicenter RCT where 474 patients undergoing live or deceased donor transplantation were stratified as per acute rejection risk. Patients with high acute rejection risk (n=139) were randomized to alemtuzumab or rabbit ATG and patients with low risk (n=335) to alemtuzumab or basiliximab (treatment with ATG or basiliximab was considered to be the conventional treatment arm). The primary endpoint of BPAR at 6 months and 12 months was significantly lower in the alemtuzumab group than in the conventional therapy group at both 6 months (3% vs. 15%, P<0.001) and 12 months (5% vs. 17%, P<0.001). However, rates of late BPAR (between 12-36 months) were higher in the alemtuzumab group compared to conventional therapy (P=0.03). Incidence of cancer-related serious adverse events was significantly higher in the alemtuzumab group versus the conventional group. Infection-related SAE and leukopenia were more common in alemtuzumab versus basiliximab group.
Date: 2015
Abbreviation: Alemtuzumab vs ATG
Comparison of alemtuzumab vs. antithymocyte globulin induction therapy in primary non-sensitized renal transplant patients treated with rapid steroid withdrawal. [Retrospective logo]
Retrospective, single-center, cohort study evaluated the cumulative incidence of one year biopsy-proven acute rejection (BPAR) among 200 consecutive primary non-sensitized kidney transplant recipients who received either alemtuzumab or rATG induction followed by rapid steroid taper, tacrolimus, and mycophenolate mofetil.
The one-yr BPAR rates were similar between the alemtuzumab and rATG groups; however, rejection Banff IA and higher was more common in the alemtuzumab arm. Alemtuzumab group had significantly higher rates of BKV nephropathy and significantly lower 3 year graft survival rate.
Date: 2016
Abbreviation: Induction in HIV
Induction Immunosuppression and Clinical Outcomes in Kidney Transplant Recipients Infected With Human Immunodeficiency Virus [Retrospective logo]
Retrospective study which analyzed the outcomes of use of rabbit ATG, IL2RA, and no induction in 830 HIV+ kidney transplant recipients & compared rates of delayed graft function, acute rejection, graft loss, and death. Compared with no induction, neither induction agent was associated with an increased risk of infection. Use of induction therapy was associated with lower rates of delayed graft function, graft loss & a trend toward lower mortality. ATG induction was associated with lower rates of acute rejection.
Date: 2017
Abbreviation: Ab Induction Therapy
Cochrane Database Syst Rev. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. [Retrospective logo]
This Cochrane database review included 99 studies (269 records; 8956 participants). Use of antithymocyte globulin (ATG) as an induction agent in kidney transplantation prevented acute graft rejection by 37%. When CNI and older non‐CNI studies were combined, an ill-sustained benefit was seen with ATG at 1 to 2 years for both all‐cause graft loss and death‐censored graft loss. ATG increased cytomegalovirus (CMV) infection, leucopenia, and thrombocytopenia but had uncertain effects on delayed graft function, malignancy, post‐transplant lymphoproliferative disorder, and new onset diabetes after transplantation. In the context of steroid minimization, alemtuzumab prevents acute rejection at 1 year compared to ATG. Rituximab had uncertain effects on death, graft loss & acute rejection.